[ad_1]
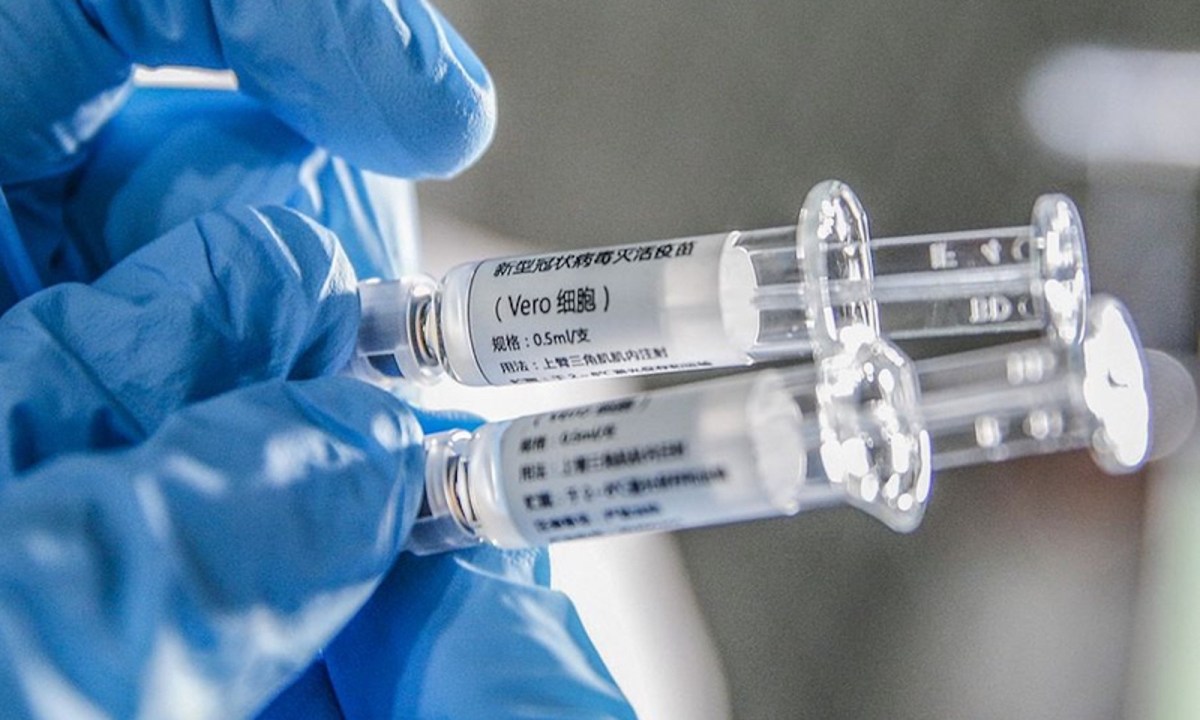
The confiscation of thousands of counterfeit Covid-19 vaccines in China has sparked new skepticism about local supplies and threatens to raise global doubts about the reliability of Chinese made doses.
Police have made at least 80 arrests in Beijing and in the eastern Shandong and Jiangsu provinces this week in a spreading scandal that harks uncomfortably to previous issues over counterfeit and dangerous locally-made products.Â
State media has predictably sought to downplay the scandal, giving scant details in their cursory reports about ongoing investigations and on how widely bogus shots are being circulated and administered. The reports have not mentioned if any officials in charge of drug development and supervision are responsible or will be sacked.Â
However, Beijing is rushing to reassure foreign nations that have ordered its approved Sinopharm and Sinovac-made vaccines that the spiraling scandal will not affect exported doses. Chinese Foreign Ministry spokesperson Wang Wenbin, in response to questions by foreign correspondents, said on Tuesday that Beijing had “notified relevant countries about the ongoing probe†and that more details would be shared.Â
Wang did not specify which countries had already been notified. But his remarks have caused some Chinese to wonder if Beijing is more forthcoming about the scandal with foreign recipient countries than with its own people. That’s adding to official transparency concerns as Beijing rolls out its inoculation programs with locally-made drugs.Â
The scandal comes as doubts swirl about the efficacy of Chinese-made vaccines as tests of the same vaccine in different countries have yielded widely divergent results. Millions of Sinopharm and Sinovac doses will soon be shipped to countries in Southeast Asia, the Middle East and South America. Spokesperson Wang stressed that Chinese vaccines had proved to be efficacious in overseas trials.Â
Reports indicate that some 80 vaccine counterfeiters are now being detained and questioned in Beijing, Jiangsu and Shandong. They reportedly sought to sell shots made of dubious ingredients as genuine ones from state-owned SinoPharm. The company’s attenuated vaccine against the novel coronavirus, developed by its subsidiary China National Biotec Group and trialed overseas, has just cleared local regulatory procedures as Beijing readies a nationwide vaccination roll-out. Â
SinoPharm’s vaccine has also been granted emergency use permits across nine nations, including the United Arab Emirates, Bahrain, Egypt, Jordan, Peru, Argentina and Morocco, after passing final-stage trials since the end of 2020, according to the drugmaker and Chinese media reports. China’s National Medical Products Administration has said the vaccine’s efficacy rate was almost 80%, much higher than the 50.4% found in trials in Brazil.Â
With emerging demand on China’s grey market by people not included in priority inoculation programs, criminal groups have apparently moved into China’s vaccine market.
Tests of the confiscated fake shots revealed that the liquid contained in syringes was saline fluid so no harm would likely be caused to anyone receiving the jabs, apart from a false sense of security. Â
Quoting a Public Security Ministry circular, Xinhua and the Beijing Daily reported that a criminal syndicate had run a workshop in Shandong since September to produce shots to be sold at a premium. It is still not clear who were the distributors and buyers and if they would be implicated.Â
Dr Tao Lina, a former immunization planning and vaccination specialist with Shanghai’s Municipal Center for Disease Control and Prevention, said on his Weibo account that China’s vaccine research and inoculation plans all gave counterfeiters loopholes to exploit and that packaging problems were also to blame.Â
“Each individual package of the SinoPharm vaccine should come with a set of unique serial numbers for authentication but the drugmaker itself may not have assigned such numbers or other security features to the batches for emergency use… Government oversight is slack as far as I know and it may be difficult for users to identify the provenance of drugs and ingredients,†wrote Tao.Â
Beijing has long been urged to strengthen quality control and law enforcement following a rash of scandals involving defective vaccines and needles over the years.Â
There was widespread ire over the government’s apparent coverup and alleged negligence in 2018 when China’s pharmaceutical and vaccine industry was roiled by lawsuits filed by the parents of children who developed severe complications after receiving rabies and diphtheria-pertussis-tetanus shots made by the listed Changchun Changsheng Biotechnology Company based in Jilin province.Â
Then, Chinese President Xi Jinping and Premier Li Keqiang gave stern-worded directives to courts to mete out heavy punishments against the firm. But critics wonder if the adverse publicity really led to any systemic changes in the industry’s supervision.
To be sure, National Medical Products Administration director Bi Jingquan was sacked that year amid the furor. But after a two-year hiatus, Bi was appointed as a deputy director of a National People’s Congress deputy-ministerial level panel on the economy and drug development in September this year.Â
[ad_2]
Source link